Corrosion, scaling and contamination – all problems that could affect the efficiency of an hvac system if water isn’t treated. We explain one approach for keeping water running free.
HVAC systems are designed to provide comfortable operating conditions for a building’s occupants. These systems require major capital investment, have long term operating costs and their energy consumption impacts significantly on the environment and profit. To maximise the return on investment and minimise the impact for the wider environment it is crucial that the structural integrity and efficiency of such systems is maintained.
The energy carrier within the majority of hvac systems is water, it has no Control of Substances Hazardous to Health (COSHH) requirement and is thus environmentally friendly, but its inherent properties and consistently variable quality present a major operational issue. The instant that water enters an hvac system it will react physically, chemically and biologically, generating processes that are interrelated and will fuel each others development. In broad terms they include, corrosion, scaling, bacterial contamination and general fouling.
Biofilms resulting from bacterial contamination for example are composed primarily of algal and bacterial microbial cells and the extra cellular polysaccharide slimes they produce. Rough or fouled surfaces provide a suitable substrate for attachment and the surrounding water bathes them in a nourishing soup encouraging colony growth. They are capable of producing basic corrosion cells and mineral acids that cause localised pitting. In addition they can provide a breeding ground for harmful bacteria given favourable conditions for growth.
The consequences of corrosion can be disproportionate to the small amounts of metal destroyed by the process. Just 2 mm of rust can reduce heat transfer by 5% across component surfaces. Scale has a more significant effect on the transfer efficiency with a layer as thin as 0·5 mm generating a 4% loss. The removal of scale and poor thermal conductivity is estimated to cost industry £1 billion per year in the UK alone with estimates as high as US$ 27 billion worldwide (Water Science and Technology Journal, April 2004). In addition scaled and corroded surfaces contribute to bacterial contamination. The insulating effect of biofilm growth is four times as resistant to heat transfer as calcium based scale.
The by-products of corrosion are problems with system components. It can block inline valves, strainers, and narrow bore pipes, reducing flow rates and increasing the rate of further sedimentation. The associated fouling will again increase the propensity for microbial growth. Impingement by particles can cause excessive wear of pumps, impellers, mechanical seals and connective pipework. Localised pitting can perforate vessels and pipes allowing system contents to escape. Inevitably this can mean plant failing.
The detrimental effects of scaling waters cause similar problems to corrosive waters and scale build up within components can be catastrophic.
Treatment regimes
When considering a water treatment regime, engineers should examine its efficacy; whole life cycle cost; how easily it can be implemented and managed; and what potential risk it presents to personnel and environment. The conventional approach to meeting these objectives has been predominantly to use chemical additives.
Corrosion inhibitors can be directed at different parts of the electrochemical process. Anodic inhibitors protect the anodic site preventing metal ions going into solution. However, underdosing an anodic inhibitor can generate localised pitting and conditions for accelerated corrosion. Cathodic inhibitors prevent electrons being removed from the metal’s surface through precipitation of a protective film at cathodic sites. Synergised inhibitors combine a mixture of anodic and cathodic inhibitors to give improved performance with reduced risk of localised pitting corrosion. Scale inhibitors work by restricting crystal germination and growth or, by shifting the pH to a more acidic solution and biofilm growth and microbial contamination are controlled using oxidising and non-oxidising additives.
Chemical additives require COSHH compliance and labelling is mandatory due to their aggressive/toxic nature and the associated risks. They are dependent on a clean system to ensure their effective dosage and it is recommended that suitable mechanical filtration accompanies their use.
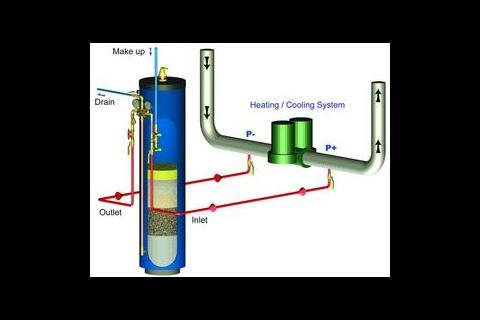
An alternative to chemical additives is the combined dolomite/calcite filter. This combines reproducible principles of water chemistry with standard water treatment technologies. The unit provides effective dirt and air separation with environmentally sound water conditioning. Processing < 1% total flow rate and filtering to < 10mm a typical unit has negligible impact on system efficiency unlike the associated pressure drop of inline filters.
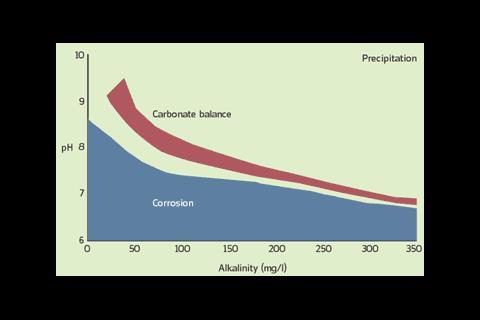
The effect of a dolomite/calcite filter is to create changes in key water parameters: pH, alkalinity and hardness. The process inhibits corrosion, controls scale formation and acts as a biocide. The unit contains a calcite/dolomite media blend that dissolves into the system water to establish carbonate equilibrium (figure 1).
The equilibrium is dynamic, relying on a saturation point. The analogy is the ability to dissolve sugar in hot tea. At saturation, excess sugar remains within the cup but if more hot water is added, the saturation point adjusts allowing the excess to be dissolved. The process is self-regulating and continuously adjusts to achieve the design chemistry buffering against system ‘top up’.
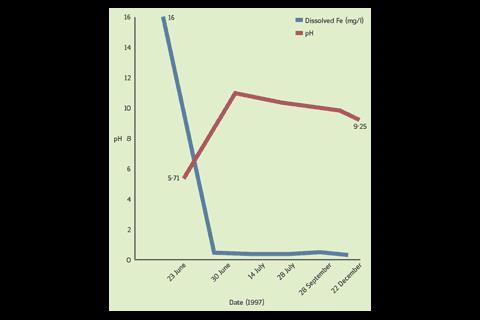
In soft water applications, hardness is elevated to prevent corrosion. The correlation between the designed water chemistry and corrosion can be seen in figure 2. This shows that pH elevates from 5·71 and stabilises at 9·25 and the level of ferrous ions decrease significantly from 16 mg/l to 0·01 mg/l and remains low. Data extrapolated from weight loss coupons immersed during a trials by Det norske Veritas (DnV) on a dolomite/calcite type filter in 1997 documented corrosion at 0 mm/year.
In hard water applications, the excess hardness is actively precipitated within the filter bed as a fine sludge that is backwashed to waste.
The test by DnV also demonstrated after six months treatment that the particle content was within the limits set for potable water.
Elevation of pH to Ä9 provides a harsh environment for bacteria as cell wall replication is difficult at pH >8·5. Unlike conventional chemical additives, the calcite/dolomite media will not contribute a nutrient source. In addition the high level of filtration actively removes nutrient load and substrates that promote colony growth. In applications where the filtration unit has replaced both the chemical inhibitors and biocide there has been no increase in total viable counts.
System efficiency
Use of a dolomite/calcite filter unit can aid greater efficiency of hvac systems. The Ringsaker municipality in Norway achieved a 6·5 % reduction in energy consumption from a relatively small heating system (4·5 m3 water volume), even with relatively clear water and low particle load before treatment. Calculations by independent energy consultant Enoksenteret compared heating season 99/00 with a 14 year archive of data seasonally adjusted for temperature variation. They achieved a payback in less than two years for the filter system from the energy savings alone.
IMAG Optical Storage used thermographic imaging of cooling fins prior to and six months post installation and documented significant improvements in fin efficiency. Uniform temperature distribution was restored across the fins and the hot spot intensity and frequency was reduced. The cooling process at its production
facility can now be controlled and the pumping requirement has been decreased by 50%. Only two of the four, 18·5 kW pumps operate to sustain the required peak cooling demand.
Installation
With a typical dolomite/calcite filter, the unit is isolated from the closed loop, flow is reversed through the media bed and collected particles are purged to waste using mains water. If contamination is high, waste can be flushed to a sediment trap, the water evaporated and the dry waste disposed of to meet effluent standards.
The backwash regime is typically phased into a monthly planned maintenance regime requiring 10 minutes/month during which 200 litres of water are consumed. Once the hvac system is cleaned the non-hazardous nature of the reaction media provides a manageable waste with negligible impact on the environment.
System water should typically be sampled at three months ensuring the unit is performing as designed.
Glen Simpson is managing director of Enwa UK. E-mail g.simpson@enwa.co.uk or visit www.enwa.co.uk
Source
Building Sustainable Design






















No comments yet