The best way of protecting a building is to ensure fire doesn’t break out in the first place. Colin Bennett describes a new system that claims to do just that
The trouble with traditional fire protection methods is that you have to have a fire before they can be used to suppress or extinguish it. Hypoxic systems are a new type of active fire protection that protects buildings and their contents by preventing a fire from occurring in the first place.
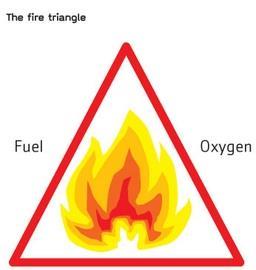
However good a conventional fire suppression system is, there will always be a certain amount of damage. In some instances, any level of damage is unacceptable – for example, where business continuity is critical or where the items being protected are irreplaceable. For a fire to occur, you need three fundamental elements – fuel, heat and oxygen. Removal of one of these will result in a fire being unable to exist. But it is not normally possible to remove an element completely, so all fire suppression systems work on the basis of reducing one or more to control/extinguish a fire.
Hypoxic systems differ in one basic respect: they are designed to prevent a fire from occurring by maintaining a low oxygen or hypoxic environment within the protected space. This is created by altering the ratio of oxygen and nitrogen.
Nitrogen makes up the majority of the atmosphere, about 78%, and is therefore readily abundant. It is a non-toxic, electrically non-conductive, inert gas and will not damage any products or equipment. The system does not rely on nitrogen being stored on site, but is produced by a nitrogen generator and compressor which minimises plant space.
Hypoxic systems typically consist of a compressor, a nitrogen generator and a control unit. The process works by drawing external ambient air into the compressor where it is compressed to 10bar or more. During this stage the air is dehumidified and cleaned, then passed through the nitrogen generator.
The generator filters the incoming air flow into two streams – an oxygen-rich air flow which is vented back to the atmosphere and a nitrogen-rich air flow which is supplied to the protected enclosure to create the hypoxic environment. The control unit constantly monitors both the generator, compressor and the protected enclosure to ensure the pre-determined oxygen concentration is maintained.
Systems can be designed to fit into the building's normal HVAC runs, removing the need for additional services to be installed within the protected enclosure, although a sensitive smoke detection process such as an aspirating system will be required as there is still a risk of pyrolysis – smoke but no open flame. The generator and control panel can either be stored within the protected area or remotely. The compressor has to be installed outside the risk area but there is virtually no limit to the distance away.
A single system can potentially protect a number of different areas. The size of the generator and compressor is not dependent upon the size of the space, but on the leakage rates from the room/building to be protected. For rooms/spaces up to 750m3, the size of the nitrogen generator can be as small as 1m3. This system has an integral nitrogen generator, compressor and control panel and can be mounted in the risk area.
The system is relatively easy and quick to install as there is no need to provide storage space for gas cylinders or water tanks. Maintenance is also relatively low and the nitrogen generators have a life of 10-15 years, with filters that need to be changed annually.
Typically, the oxygen concentration within the protected space is maintained at about 15% but this depends on the materials being stored. The space can be occupied by staff. An atmosphere containing 15% oxygen is similar to the environment in aircraft cabins.
In the UK, the HSE regards spaces with reduced oxygen levels as confined spaces and therefore safe systems of work and management procedures need to be established to monitor people and movement within them. However, for oxygen levels of 15%, people
are able to work for about four hours before they need to take a break. If spaces are to be unoccupied or the occupancy is short-term, oxygen levels could be reduced to about 10-12%. Again this depends on the materials being protected.
Hypoxic systems are ideal for:
- IT and communications rooms, where gas suppression systems are typically installed. The use of hypoxic systems in this instance can significantly reduce the amount of services required, ie storage of cylinders, pressure relief dampers etc, not to mention the consequences of false alarms associated with gas suppression systems.
- Warehouses and storage buildings, where even with sprinkler systems a fire can cause a lot of damage to stock. With a hypoxic system, there are fewer restrictions with regards to the types of goods stored and racking/storage configurations, allowing a greater flexibility in the use of the building. Cold-storage spaces, and other areas that are difficult to protect with sprinklers, can be more easily protected by a hypoxic system.
- Museums, libraries and heritage buildings, where the contents are irreplaceable.
What is a hypoxic environment?
Typically, dry air is made up of about 21% oxygen, 78% nitrogen and 1% of other gases such as carbon dioxide and argon. This ratio of gases is independent of altitude – it is the same whether you are at sea level or at the top of Mount Everest.
However, as the altitude increases, the air pressure decreases, creating a hypobaric environment. At sea level the air pressure is normally approximately 101kPa and the partial pressure of oxygen is about 21kPa. This is referred to as normobaric, normoxic air.
At higher altitudes, although the air is still made up of 21% oxygen, the partial pressure of the oxygen is reduced and is referred to as hypobaric, hypoxic air. For example, at 4000m the partial pressure of oxygen is about 13kPa, which is equivalent to an oxygen concentration of about 13% at sea level.
A hypoxic system creates a normobaric, hypoxic environment where the oxygen concentration is reduced but the air pressure remains normal and is achieved by replacing the oxygen with nitrogen.
However, the question remains: if a candle can burn at high altitude, why does it not do so when placed within an enclosure protected by a hypoxic system with an equivalent oxygen concentration? The answer is that the increased density of the nitrogen molecules obstructs the availability of the oxygen molecules needed for combustion to occur.
Downloads
Key components of a hypoxic system
Other, Size 0 kbSchematic diagram of Wagner’s OxyReduct system
Other, Size 0 kb
Source
Building Sustainable Design
Postscript
Colin Bennett is a senior engineer with Cundall Fire Engineering and can be contacted at c.bennett@cundall.com




















No comments yet